Buy Online Cheap Generic Glucophage - Safe, Affordable Guide
October 5 2025CGMP Compliance: What It Means for Your Medications and Why It Matters
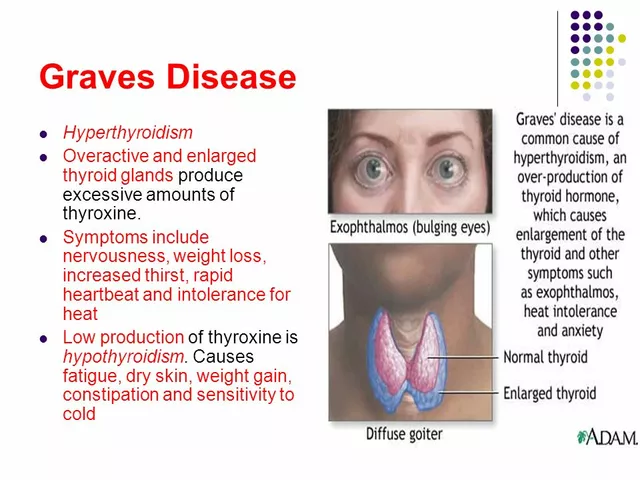
When you take a pill, you expect it to work the way it should—no surprises, no contamination, no fake ingredients. That’s not luck. It’s CGMP compliance, Current Good Manufacturing Practices, the set of rules that ensure drugs are consistently produced and controlled according to quality standards. Also known as good manufacturing practices, it’s the invisible guardrail keeping your medicine safe from factory errors, dirty equipment, or rushed production. Without it, a batch of antibiotics could be weak, a blood thinner could be too strong, or worse—you could get a pill with the wrong chemical entirely.
CGMP compliance isn’t just paperwork. It’s hands-on control at every step: from how raw ingredients are tested, to how machines are cleaned between batches, to how workers are trained to spot a mislabeled bottle. It’s why the FDA can trace a single pill back to the exact day and shift it was made. It’s why companies can’t just slap a label on whatever comes out of a machine. This system directly connects to drug supply chain security, the network of checks that prevent counterfeit drugs from reaching pharmacies, and pharmaceutical serialization, the unique barcodes on every package that let regulators track where a drug has been. These aren’t buzzwords—they’re the tools that make CGMP real.
And it’s not just about big pharma. Even generic drugs, which save billions each year, must meet the same standards. That’s why ANDA, Abbreviated New Drug Applications, the path for generics to get FDA approval requires proof of CGMP compliance before a single tablet is sold. If a factory fails an inspection, the FDA can shut it down. No exceptions. That’s why quality assurance isn’t just a department—it’s the foundation of trust between you and every medication you use.
What you’ll find in the posts below are real stories of how CGMP compliance shows up in everyday medicine: from how traceability stops counterfeit drugs, to why a tiny labeling mistake can trigger a recall, to how manufacturing fears are changing how drugs are made today. These aren’t theory pieces—they’re about what happens when rules work, and what goes wrong when they don’t. Whether you’re a patient, a caregiver, or just someone who wants to know their meds are safe, this collection gives you the facts behind the bottle.
 3 Dec
3 Dec
FDA Facility Inspections: How the Agency Ensures Quality in Manufacturing
The FDA ensures product quality through risk-based facility inspections that check compliance with manufacturing standards. Learn how inspections work, what they look for, and how to prepare to avoid costly failures.
Read More...