How Insurers Choose Which Generics to Cover: The Real Rules Behind Formulary Decisions
January 13 2026CAPA: Understanding Corrective and Preventive Actions in Pharma Quality
When a medicine doesn’t work right, or a batch gets contaminated, or a pharmacy keeps mislabeling pills—CAPA, Corrective and Preventive Action. It’s the formal process used to fix what went wrong and stop it from happening again. This isn’t paperwork for the sake of paperwork. CAPA is the difference between a safe drug reaching your shelf and a recall that puts lives at risk. It’s built into every major pharmaceutical operation—from the factory floor to the hospital pharmacy—and it’s one of the top things the FDA checks during inspections.
Corrective Action, the immediate fix for a problem that already happened might mean throwing out a bad batch of pills or retraining staff who mixed up labels. But Preventive Action, the step that stops the problem before it happens again is where real change lives. That could mean redesigning a workflow, adding a second check for high-alert drugs, or updating software to flag dangerous abbreviations like "QD" or "U"—the same ones that cause errors in prescriptions. CAPA doesn’t ignore root causes. It digs into them. Why did the machine break? Why did the person miss the warning? Why did the training fail? Without this depth, you’re just putting band-aids on bullet wounds.
Look at the posts here. You’ll see CAPA in action—whether it’s in FDA facility inspections, where inspectors look for evidence that companies aren’t just fixing mistakes but preventing them. Or in quality assurance concerns from 2025, where brand trust hangs on whether a company can prove it learned from past failures. It’s behind medication errors in hospitals when double-check protocols are added after a near-miss. It’s in the generic drug switch studies where doctors track if patients had bad reactions after switching—and then change how substitutions are handled. CAPA isn’t a department. It’s a mindset. It’s what turns a mistake into a lesson, and a lesson into a system that protects people.
What you’ll find below isn’t theory. It’s real cases, real data, and real consequences. From how regulatory capture can weaken CAPA systems to how manufacturing fears are forcing companies to build better checks, every post here shows how CAPA keeps the system alive. Whether you’re a pharmacist, a patient, or just someone who cares about what’s in your medicine, understanding CAPA means understanding how safety is built—not by luck, but by design.
 6 Dec
6 Dec
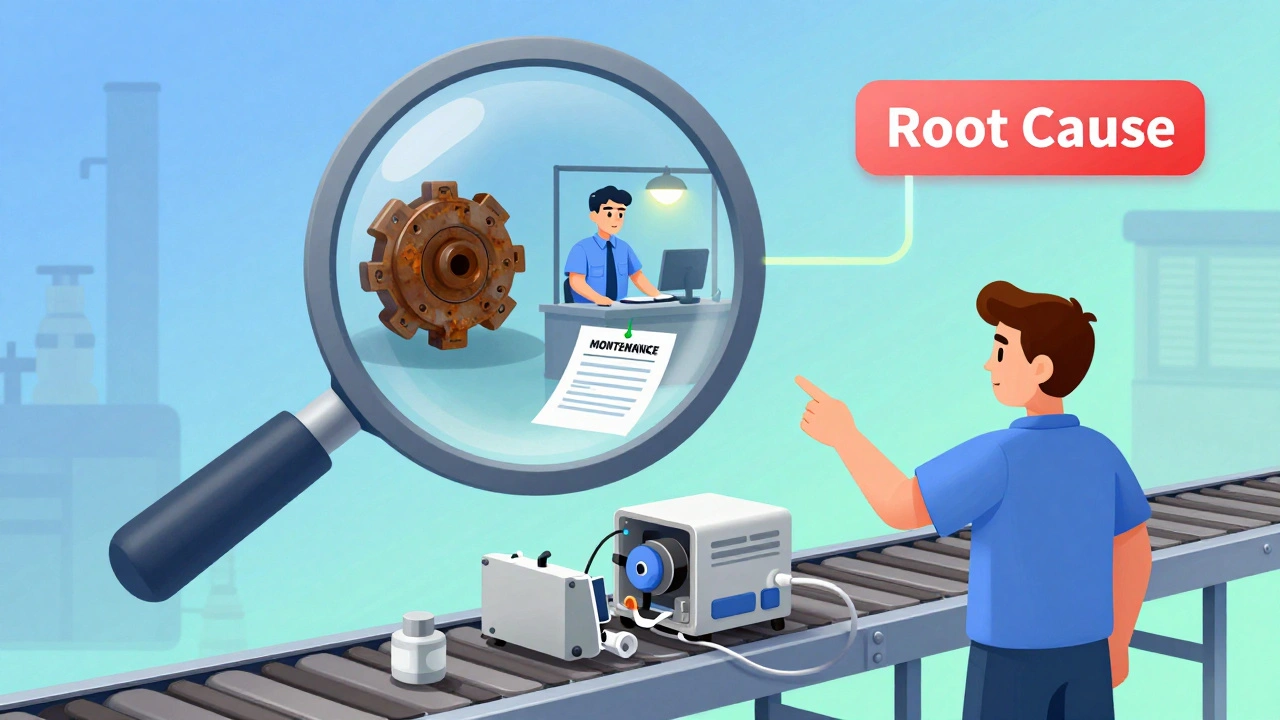
How Manufacturers Fix Quality Problems: A Practical Guide to Corrective Actions
Manufacturers fix quality problems through structured corrective actions that target root causes, not just symptoms. Learn how CAPA systems work, why most fail, and how to implement them effectively in regulated industries.
Read More...