When a factory produces a batch of medical devices and 12 out of 1,000 units fail inspection, what happens next? Stopping the line and tossing out the bad parts isn’t enough. That’s a correction-a quick fix. What manufacturers actually need is a corrective action: a deep, documented, and verified process to make sure those 12 failures never happen again. This isn’t just paperwork. It’s how companies survive audits, keep customers, and avoid recalls.
What’s the Difference Between a Correction and a Corrective Action?
A correction is like putting a bandage on a cut. You fix the immediate problem. Maybe you rework a misaligned part. Maybe you recalibrate a machine. Done. The product is fixed. But the cause? Still there. A corrective action digs under the skin. It asks: Why did the part misalign? Was it the tool? The operator? The temperature in the room? The maintenance schedule? It’s not about fixing the part-it’s about fixing the system that made the bad part. The FDA and ISO 13485 don’t just recommend this. They require it. In medical device manufacturing, skipping real corrective action isn’t a mistake-it’s a violation. And it shows up fast. In 2022, 43% of FDA warning letters cited inadequate corrective actions. That’s not a glitch. That’s a pattern.The Six-Step Corrective Action Process
Every effective corrective action follows the same six steps. It’s not magic. It’s method. And it works.- Identify the problem - This starts with data. Not gut feeling. Not a supervisor’s complaint. It’s when a quality control check shows a defect rate above the control limit. Or when a customer returns a batch. Or when a sensor on the assembly line flags an anomaly. The trigger is clear: something is out of spec.
- Evaluate and categorize - Not all problems are equal. A cracked casing on a pacemaker? Critical. A slightly off-color label? Minor. Manufacturers use risk scoring to decide how hard to dig. FDA’s cGMP rules say: if it affects patient safety, you go full CAPA. If it’s cosmetic? A simple correction might do.
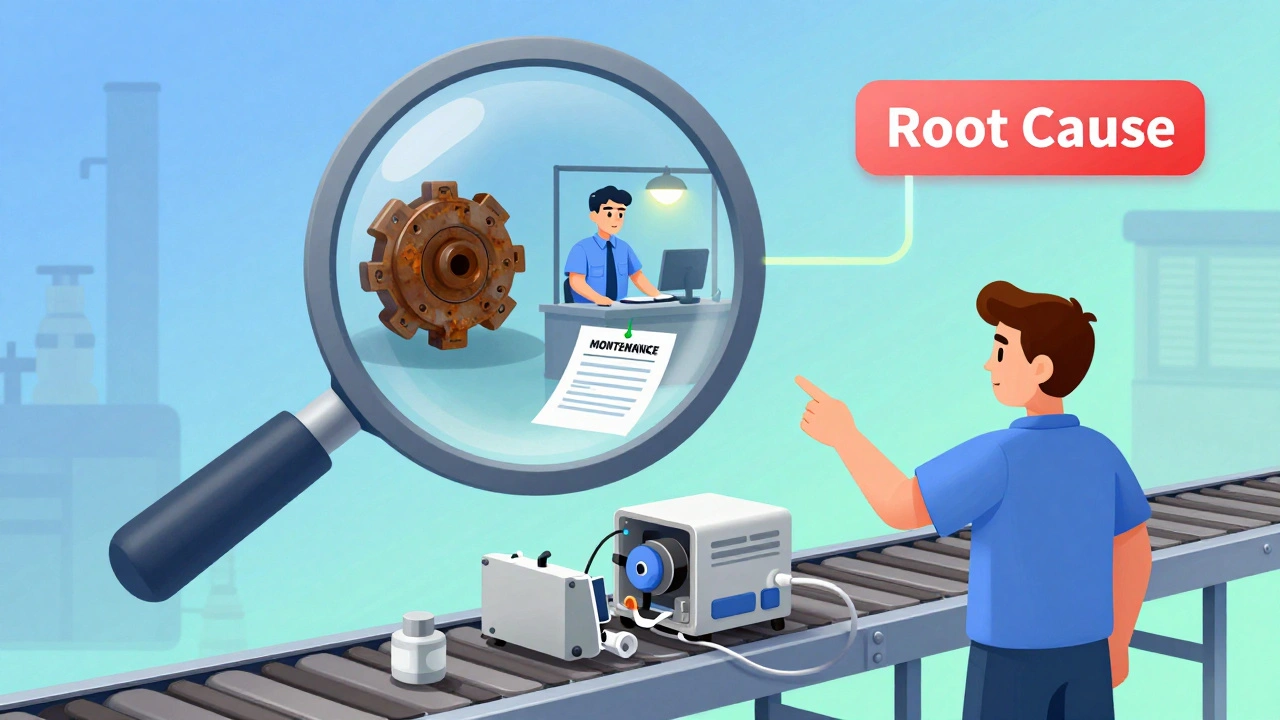
- Find the root cause - This is where most fail. Too many teams jump to “operator error” and call it done. But why did the operator make that mistake? Was the training outdated? Was the tool hard to use? Was the work station poorly lit? The 5 Whys method helps. Ask “why” five times. Fishbone diagrams map out possible causes: people, machines, materials, methods, environment. Real root causes are rarely obvious. They’re hidden in shift changes, supplier batches, or software updates.
- Plan the fix - Once you know the cause, you design a solution. Not a guess. A plan. This includes:
- Specific actions (e.g., “Rewrite machine calibration procedure,” “Install torque sensor on assembly station”)
- Who does what (named person, not “quality team”)
- Deadlines (e.g., “Complete training by March 15”)
- How you’ll know it worked (e.g., “Defect rate must drop below 0.5% for 3 consecutive production cycles”)
- Implement the fix - Time to act. Train staff. Update SOPs. Change the machine settings. Replace the supplier. The fix isn’t real until it’s in use. And it must be documented. Every step. Every signature. Every change log.
- Verify effectiveness - This is the make-or-break step. Did the fix actually work? You can’t just hope. You test. You collect data. You compare defect rates before and after. Sample sizes matter. For process validation, you need at least 30 units. You check calibration records. You audit the new procedure. If the defect rate didn’t drop? You didn’t fix the root cause. You go back to step three.
Why Do So Many Corrective Actions Fail?
The data doesn’t lie. According to FDA inspection reports, 61% of companies fail because they fix symptoms, not causes. Here’s why:- Too much paperwork, not enough action - Some CAPA systems generate 47 pages of documents per issue. Quality teams spend hours filling forms instead of fixing machines. The result? Delays. Frustration. Workarounds.
- No ownership - When “the quality department” is responsible, no one is. Successful CAPAs assign one person to own each action. Not a team. Not a role. A name.
- No verification - They implement. They close the ticket. They never check if it stuck. A fix that isn’t verified is just a guess dressed up as a policy.
- Too slow - Root cause analysis takes time. On average, 8.4 hours per major issue. Companies that rush it get shallow answers. And repeat failures.
What Works: Real Examples from the Floor
One automotive supplier had a 2.8% defect rate on brake calipers. They tried retraining. They tried reworking. Nothing stuck. Then they used a fishbone diagram. Turned out, the hydraulic press was overheating during third shift. The cooling system hadn’t been cleaned in 18 months. The maintenance log showed “no issues.” They cleaned the system, added a temperature alarm, and trained night shift staff to check it. Within 18 months, defects dropped to 0.4%. The fix? A $300 cleaning kit and a 15-minute daily check. A medical device maker in Germany had recurring contamination in sterile packaging. Their CAPA said “improve cleaning.” But the real cause? A new supplier of sterilization bags. The new bags had a different weave. Moisture got trapped. The fix? Change the supplier back-and add a material spec to the procurement checklist. Simple. But only because they dug deep.Technology Is Changing How CAPA Works
The old way? Paper forms. Excel sheets. Email chains. The new way? Digital systems that connect to machines. Manufacturers using AI-powered tools can now detect patterns before defects happen. If a machine’s vibration pattern shifts by 7%, the system flags it-not because it failed, but because it’s trending toward failure. That’s not corrective action. That’s preventive. But it’s built on the same foundation. Tulip’s 2023 data shows companies using digital CAPA systems cut documentation time by 41%. They also improved verification speed by 33%. The FDA’s 2023 Digital Health Plan encourages this. Blockchain audit trails, real-time data feeds, automated alerts-they’re not future tech. They’re becoming standard.
Who Needs This the Most?
Not every manufacturer needs a full CAPA system. But if you’re in these industries, you have no choice:- Medical devices - ISO 13485 requires it. 82% of firms use formal CAPA.
- Pharmaceuticals - cGMP rules demand documented corrective actions for any deviation affecting safety.
- Aerospace - AS9100 standards require traceability and verification.
- Automotive - IATF 16949 demands root cause analysis for all customer complaints.
What to Do If You’re Starting From Scratch
You don’t need a $500,000 software system. Start here:- Pick one recurring problem. Not the biggest. Not the most expensive. Just one you can fix in 30 days.
- Assemble a team: one operator, one engineer, one quality rep, one supervisor.
- Use the 5 Whys. Write down every answer. Don’t stop until you hit something you can change.
- Write a one-page plan: What? Who? When? How will we know it worked?
- Do it. Track results for two weeks.
- Document it. Share it. Make it a standard.
Final Thought: It’s Not About Compliance. It’s About Control.
Corrective actions aren’t there to please auditors. They’re there to give you control. Control over your process. Control over your quality. Control over your reputation. When you fix the root cause, you stop fighting the same fire over and over. You stop wasting time on rework. You stop losing customers. And you stop getting warning letters. The best manufacturers don’t just fix problems. They build systems that prevent them. And that’s not just good quality. That’s good business.What’s the difference between corrective action and preventive action?
Corrective action fixes problems that have already happened. Preventive action stops problems before they occur. For example, if a machine breaks down weekly, corrective action fixes the broken part. Preventive action installs a sensor that alerts you when the motor starts overheating-before it breaks. Preventive action is proactive. Corrective action is reactive. Both are needed, but they serve different purposes.
How long should a corrective action take to complete?
There’s no fixed timeline, but speed matters. Minor issues should be closed in under 30 days. Major ones-like those affecting safety-should be resolved within 90 days. The key isn’t speed alone. It’s thoroughness. Rushing root cause analysis leads to repeat failures. The FDA expects timely action, but not rushed action. A 60-day CAPA with solid verification beats a 10-day CAPA that doesn’t work.
Do all quality issues require a formal CAPA?
No. Only issues with a root cause that can be eliminated to prevent recurrence need a formal CAPA. Minor, one-time issues-like a misprinted label on a single batch-can be handled as a correction. But if the same issue happens twice, or if it affects safety, performance, or compliance, you need a CAPA. The rule of thumb: if you’re seeing it more than once, it’s not a fluke. It’s a system problem.
What happens if a corrective action fails?
If the fix doesn’t work, you don’t close the CAPA. You reopen it. You go back to root cause analysis. The failure is data. It tells you your first assumption was wrong. Maybe the root cause wasn’t the machine-it was the training. Or the supplier. Or a change in raw material. A failed CAPA isn’t a failure of the system. It’s a sign the system is working: it’s catching the gap. The only real failure is pretending it’s fixed when it’s not.
Can small manufacturers afford a full CAPA system?
Yes. You don’t need expensive software. Start with a simple spreadsheet. Use free templates from ISO or FDA websites. Focus on one process at a time. A small medical device maker with 15 employees can run a full CAPA system with just one person managing it. The cost isn’t in tools-it’s in time. And the cost of not doing it? Recalls, lawsuits, lost certifications. That’s far more expensive.





Wesley Phillips
December 7, 2025 AT 17:02