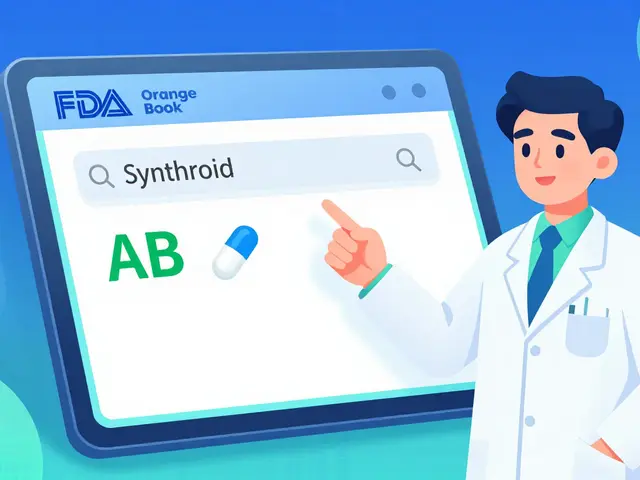
How to Verify the FDA Orange Book for Generic Equivalence
January 12 2026Pharmaceutical Serialization: What It Is and Why It Matters for Drug Safety
When you pick up a bottle of medicine, you expect it to be real, safe, and exactly what your doctor prescribed. But counterfeit drugs are a growing problem—fake pills, tampered packages, and stolen inventory can slip into the supply chain. That’s where pharmaceutical serialization, the system of assigning unique identifiers to every drug package so it can be tracked from manufacturer to patient. Also known as drug traceability, it’s now required by law in the U.S., EU, and many other countries to protect public health. This isn’t just a tech buzzword—it’s a lifeline for patients who might otherwise get a deadly fake version of their heart medication, antibiotics, or insulin.
Serialization works by putting a unique barcode or RFID tag on each unit—like a digital fingerprint for your pill bottle. Every time that package moves—through a distributor, a warehouse, or into a pharmacy—it’s scanned and recorded. If something looks off, like a duplicate code or a package that never left the factory, the system flags it. This helps catch stolen drugs, illegal imports, and counterfeiters before they reach you. The FDA serialization, the U.S. regulatory framework requiring unique product identifiers on prescription drug packages is one of the strictest in the world, and it’s forcing manufacturers to rethink how they package and ship medicines. Meanwhile, supply chain security, the broader effort to protect medicines from tampering, theft, and fraud at every stage relies on serialization as its backbone. Without it, tracking a single recalled batch across thousands of pharmacies would be impossible.
What you’ll find in these articles isn’t just theory—it’s real-world stories of how serialization catches fakes, why some manufacturers struggle with the tech, and how patients benefit when the system works. You’ll read about how a single mislabeled package can trigger a nationwide alert, how pharmacies verify drugs before handing them over, and why even small errors in coding can lead to costly recalls. These posts cover the technical side, the human impact, and the legal rules that make it all happen. Whether you’re a pharmacist checking a shipment, a patient worried about counterfeit drugs, or just someone who wants to know their medicine is safe, this collection gives you the facts without the jargon.
 1 Dec
1 Dec
DSCSA Track-and-Trace: How the U.S. Is Stopping Counterfeit Drugs Before They Reach You
The DSCSA track-and-trace system is the U.S. government's latest defense against counterfeit drugs. By requiring unique serial numbers and electronic tracking at every step, it’s making the pharmaceutical supply chain safer than ever before.
Read More...