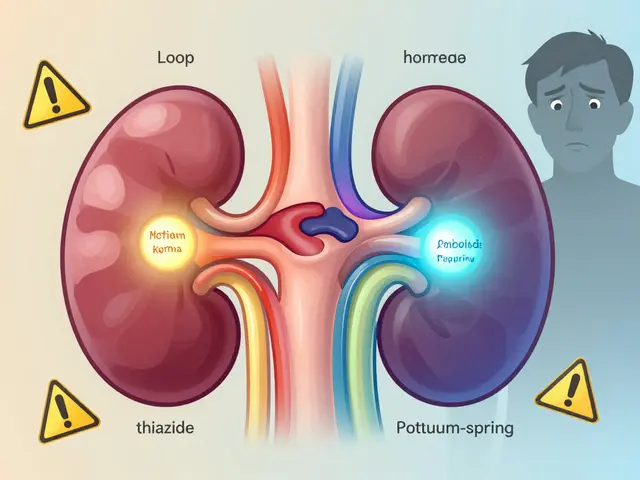
Diuretics: Understanding Electrolyte Changes and Dangerous Drug Interactions
December 27 2025ANDA: What It Is, Why It Matters for Generic Drugs, and How It Shapes Your Medicine
When you pick up a generic pill at the pharmacy, chances are it got there through an ANDA, Abbreviated New Drug Application, the FDA’s official pathway for approving generic versions of brand-name drugs. Also known as the generic drug application, it’s the backbone of affordable medicine in the U.S. Unlike the long, expensive process brand-name companies go through, ANDA lets manufacturers prove their version works the same way—without repeating every clinical trial. The FDA doesn’t need to retest safety from scratch. Instead, they check if the generic matches the original in active ingredient, strength, dosage form, and how it’s absorbed by your body. If it passes, the drug gets approved and hits shelves at a fraction of the cost.
This system isn’t just about saving money—it’s about access. Without ANDA, drugs like metformin, lisinopril, or simvastatin would still cost hundreds a month. And while some people worry generics aren’t the same, the FDA requires them to be bioequivalent, meaning they deliver the same amount of medicine into your bloodstream at the same rate. That’s why studies on drugs like warfarin and levothyroxine show that switching to generics under proper monitoring works just as well. But ANDA isn’t perfect. It’s why some patients feel worse on generics—not because of chemistry, but because of psychology. It’s also why quality control in manufacturing matters so much. If a plant fails an FDA inspection, the ANDA can be pulled. That’s why posts about FDA inspections, the agency’s risk-based checks on drug manufacturing facilities and DSCSA track-and-trace, the system that follows every pill from factory to pharmacy tie directly into ANDA. One bad batch, one mislabeled bottle, and the whole approval can come under fire.
ANDA also connects to how insurance treats generics. If a drug has an approved ANDA, it’s usually on formularies. But if your insurer denies coverage for a brand-name drug, you might need to appeal—and that’s where insurance denial appeal, the process of challenging a refusal to cover a prescribed medication comes in. Even the debate over generic drug classifications, how drugs are grouped by therapeutic use, legal schedule, or insurance tier starts with ANDA. It’s the reason two identical pills can be in different categories. And when cultural beliefs affect whether people trust generics, it’s often because they don’t understand what ANDA actually means. That’s why posts on multicultural perspectives on generics, how language, color, and tradition shape medication adherence matter. This isn’t just paperwork—it’s your health on the line.
Behind every generic you take is a stack of data, a factory inspection, a bioequivalence study, and a regulatory decision. ANDA is the quiet engine that keeps medicine affordable. But it’s only as strong as the systems around it—quality control, supply chain tracking, and patient trust. Below, you’ll find real-world stories and data that show how this system works, where it fails, and what you need to know to make smarter choices about your prescriptions.
 1 Dec
1 Dec
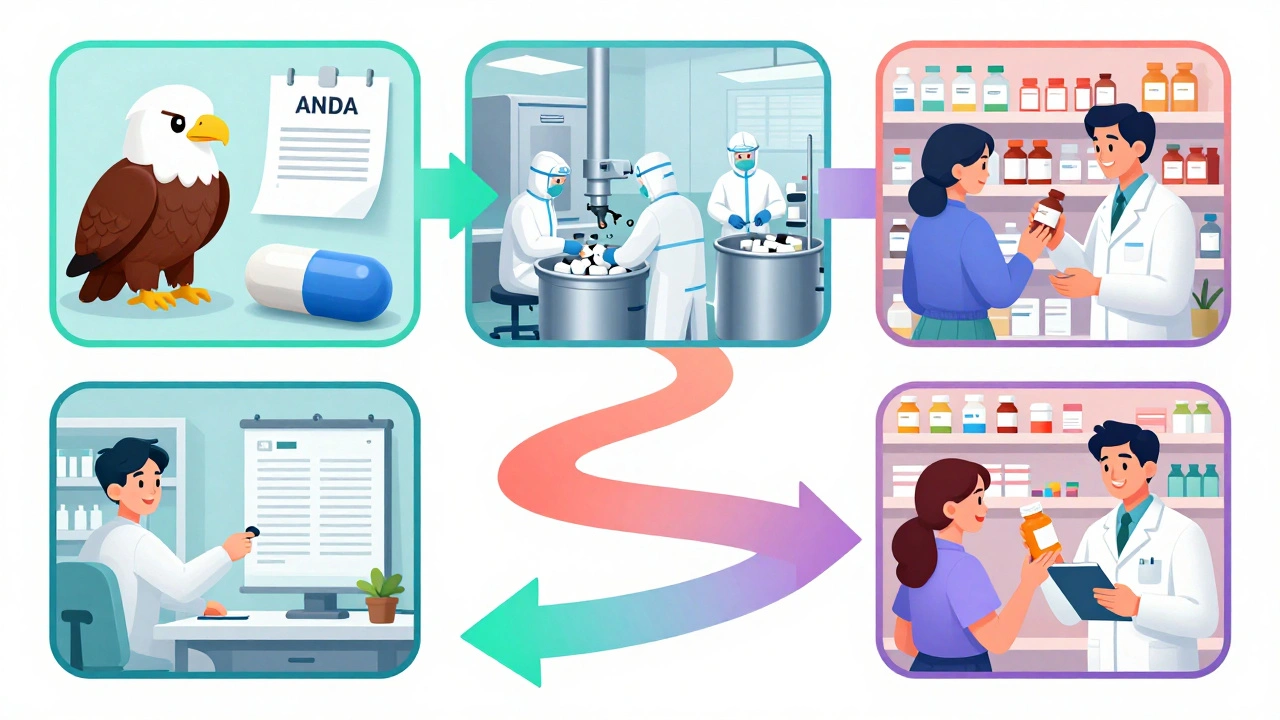
From ANDA to Shelf: How Generic Drugs Reach Retail Pharmacies
Discover how generic drugs move from FDA approval through manufacturing, payer negotiations, and distribution to reach retail pharmacies-and why this process saves Americans billions each year.
Read More...