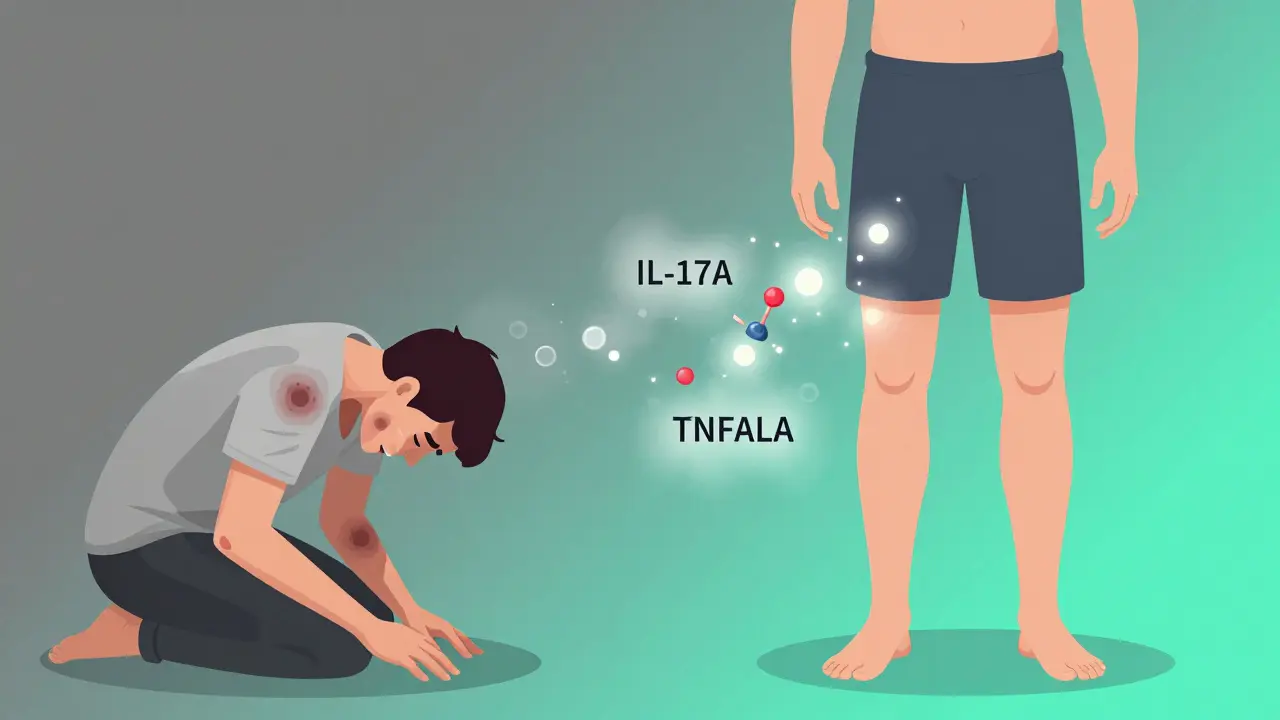
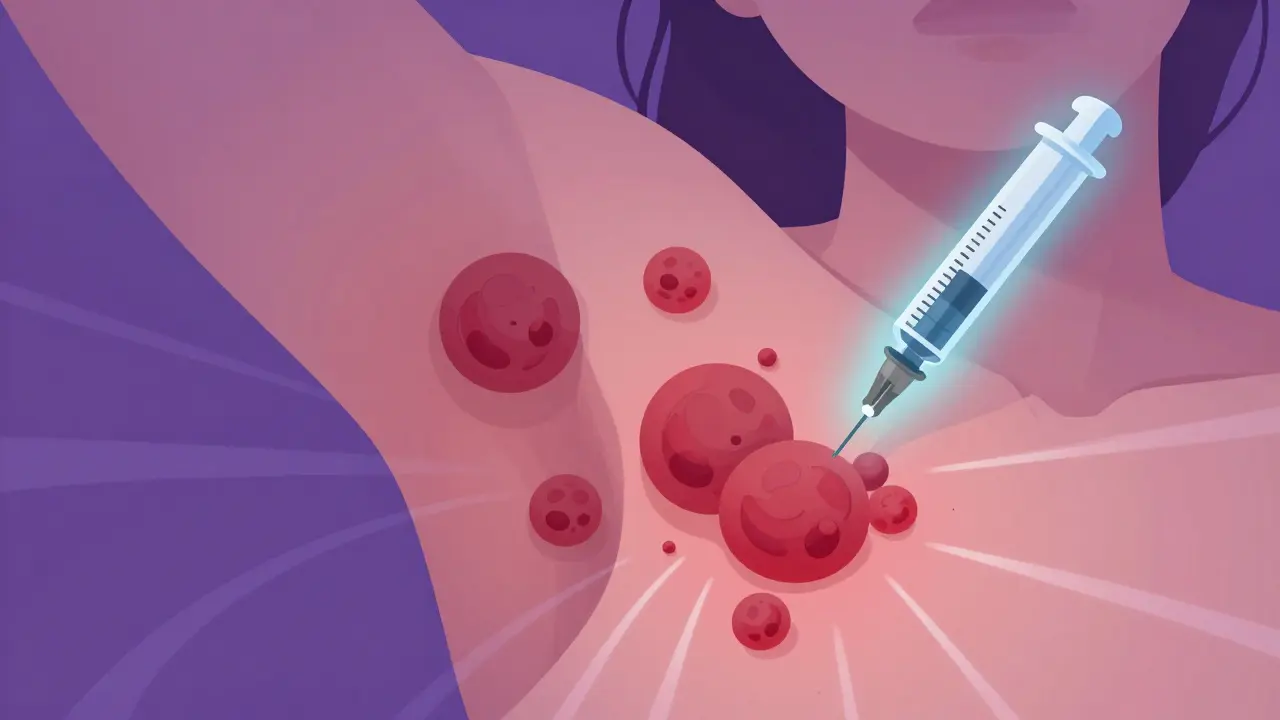
Hidradenitis suppurativa isn’t just a skin rash. It’s a chronic, painful condition that turns hair follicles into deep, recurring abscesses-often in the armpits, groin, or under the breasts. These aren’t pimples. They’re fistulas, tunnels under the skin, oozing pus and blood, and they don’t go away with soap or antibiotics. For many, it’s a daily battle with pain, odor, and isolation. Until recently, the only real options were surgery or steroids, both harsh and often temporary. Now, biologic therapy is changing everything.
What Exactly Is Hidradenitis Suppurativa?
HS starts when hair follicles get blocked-not by dirt, but by thickened skin cells. That blockage triggers inflammation deep in the skin, forming hard, painful lumps called nodules. These swell, burst, and then heal poorly, leaving scars and tunnels under the skin. It’s not contagious. It’s not caused by poor hygiene. It’s an autoimmune disease where the body attacks its own tissue.
Three out of every four people with HS are women. It usually shows up between ages 20 and 29. Obesity and smoking make it worse, but even thin, non-smokers get it. The pain is constant. Many describe it as a deep, throbbing ache that makes sitting, walking, or wearing tight clothes unbearable. Some patients miss work for weeks. Others stop dating or going to the gym.
Doctors use the Hurley staging system to grade severity:
- Stage I: Single or few abscesses, no scarring or tunnels.
- Stage II: Recurrent abscesses, with tunnels forming between them.
- Stage III: Widespread, connected tunnels and abscesses across large areas.
Biologics are now recommended as first-line treatment for Stage II and III HS-when conventional treatments like antibiotics or hormonal pills have failed.
How Biologic Therapy Works
Biologics are made from living cells, not chemicals. They’re designed to block specific parts of the immune system that drive inflammation. In HS, the body overproduces proteins like TNF-alpha, IL-17A, and IL-17F. These proteins act like alarm bells, telling immune cells to attack the skin.
Biologics silence those alarms. They’re injected under the skin-usually once or twice a week at first, then every few weeks. Unlike oral drugs that flood your whole body, biologics target just the troublemakers. That means fewer side effects… but not zero.
Three biologics are currently FDA-approved for HS:
- Adalimumab (Humira): Blocks TNF-alpha. First approved in 2015. Given as 40 mg weekly or every other week.
- Secukinumab (Cosentyx): Blocks IL-17A. Approved in 2024. Dosed at 300 mg weekly for 5 weeks, then monthly.
- Bimekizumab (BIMZELX): Blocks both IL-17A and IL-17F. Approved in June 2024. Dosed at 320 mg every 4 weeks after a higher starting dose.
Each works differently. Adalimumab has been around longest, so we know more about it. Secukinumab and bimekizumab are newer but show stronger results in early trials.
How Effective Are They?
Success is measured by HiSCR50-meaning at least half the abscesses and nodules are gone, with no new ones forming. Here’s what the data shows:
| Biologic | Target | HiSCR50 at Week 12-16 | Placebo Response | Week 52 Response |
|---|---|---|---|---|
| Adalimumab | TNF-alpha | 41.8% | 26.0% | 48.7% |
| Secukinumab | IL-17A | 44.5% | 20.5% | 56.4% |
| Bimekizumab | IL-17A & IL-17F | 66.9% | 28.0% | Not yet reported |
Bimekizumab stands out. In one trial, nearly two-thirds of patients saw half their lesions clear in just 16 weeks. That’s better than any previous treatment. Secukinumab holds up longer-more patients stay in remission at one year. Adalimumab works well but often needs higher doses over time.
Real-world feedback from patient forums like MyHSteam and Reddit confirms this. About 68% of people on adalimumab report fewer painful nodules within 12 weeks. On secukinumab, 56% say their abscesses “disappeared” within a month. But side effects happen: injection site redness, fatigue, or upper respiratory infections. About one in three patients stop because of cost or side effects.
Cost, Access, and Insurance
These drugs are expensive. In the U.S., monthly prices are:
- Adalimumab: $5,800
- Secukinumab: $6,200
- Bimekizumab: $6,900
Most patients pay $1,000-$1,500 out-of-pocket even with insurance. Medicaid approval rates are under 50%. Private insurers are better, but prior authorizations take weeks. Some pharmaceutical companies offer patient assistance programs-$0 co-pays for those who qualify.
Outside the U.S., access varies. In the UK, NHS guidelines now include biologics for HS, but waiting lists can be long. In Canada and Australia, approval is possible but requires specialist referral and proof of treatment failure.
Who Benefits Most-and Who Doesn’t
Biologics work best when started early. If you have deep tunnels and scarring (Stage III), they can reduce pain and new lesions, but they won’t erase old scars. Surgery is still needed to remove damaged tissue.
Patients with mostly inflamed nodules and few tunnels respond best to IL-17 inhibitors like secukinumab and bimekizumab. Those with long-standing, scarred disease often do better with adalimumab.
Not everyone responds. About 20-30% of patients see little to no improvement. That’s why doctors test response at 12 weeks. If you’re not getting better, they’ll switch you to another biologic-often from a different class.
There are also risks. All biologics increase infection risk. You must be screened for tuberculosis and hepatitis before starting. People with heart failure or multiple sclerosis should avoid them. Some report mild depression or headaches. Serious side effects are rare-about 12% of patients stop due to adverse events.
What Happens After Starting?
It’s not a cure. You’ll need to keep taking the drug. Stopping usually means symptoms return within months.
Doctors monitor you closely:
- Every 12 weeks: Check skin with IHS4 scoring (counts abscesses and nodules).
- Every 3 months: Blood tests for liver function, cholesterol, and inflammation markers.
- Every 6 months: Full physical exam and infection screening.
Interestingly, many patients see their lipid profiles improve-triglycerides drop, HDL (good cholesterol) rises. That’s a bonus. HS patients have higher heart disease risk. Biologics may be protecting their hearts too.

What’s Next? New Treatments on the Horizon
Three more biologics are in late-stage trials:
- Guselkumab: Blocks IL-23. Shows 58% HiSCR50 in early trials.
- Spesolimab: Targets IL-36. Promising for patients who didn’t respond to other biologics.
- TAK-279: A TYK2 inhibitor. Taken as a pill-could be a game-changer if approved.
Researchers are also testing combinations: biologics + surgery, or biologics + lifestyle changes. One 2024 study showed 89% of patients on bimekizumab + surgical removal of tunnels reached HiSCR50-far higher than biologic alone.
Future plans include blood tests to predict who will respond to which drug. A 2024 study identified a 12-gene signature that predicts adalimumab response with 85% accuracy. Personalized treatment is coming.
Real Talk: What Patients Say
On Reddit, one woman wrote: “I went from wearing baggy clothes all summer to wearing shorts for the first time in 10 years after six months on secukinumab. I cried in the pharmacy.”
Another man on MyHSteam said: “I lost 40 pounds after starting biologics-not because I was trying to, but because I could finally walk again.”
But the pain isn’t gone for everyone. One user shared: “I’ve been on adalimumab for 18 months. My nodules are better, but I still have drainage. I’m on my third insurance denial this year.”
The common thread? Early action. Patients who start biologics before scarring sets in have the best outcomes. Smoking cessation and weight loss help too-no substitute for medication, but they make it work better.
Final Thoughts
Hidradenitis suppurativa used to be a diagnosis no one talked about. Now, it’s a treatable disease. Biologics aren’t perfect. They’re expensive. They require lifelong commitment. But for the first time, there’s real hope.
If you have recurring, painful lumps in your armpits, groin, or under your breasts-don’t wait. Don’t think it’s just folliculitis. See a dermatologist who knows HS. Ask about biologics. Bring your Hurley stage. Ask for a referral to a specialist clinic. The longer you wait, the harder it gets to reverse the damage.
HS doesn’t define you. But with the right treatment, you can live beyond it.
Can biologic therapy cure hidradenitis suppurativa?
No, biologic therapy does not cure hidradenitis suppurativa. It controls the inflammation that causes painful nodules and abscesses. Most patients need to stay on treatment long-term to keep symptoms under control. Stopping the medication usually leads to a return of symptoms within months.
How long does it take for biologics to work for HS?
Most patients start seeing improvement in 4 to 12 weeks. Some notice less pain and fewer new lumps within a month, especially with secukinumab or bimekizumab. Full results typically take 12 to 16 weeks. Doctors usually wait until week 12 to decide if the treatment is working before switching.
Are biologics safe for long-term use?
Biologics are generally safe for long-term use under medical supervision. The main risks are increased susceptibility to infections like tuberculosis or pneumonia. Regular blood tests and screenings help manage these risks. Some patients experience mild side effects like injection site reactions or fatigue. Serious side effects are rare, affecting fewer than 1 in 10 users.
Why are biologics so expensive for HS treatment?
Biologics are complex to manufacture, requiring living cells and precise lab conditions. They’re also protected by patents, limiting competition. In the U.S., prices are set by pharmaceutical companies without government price controls. Insurance coverage varies widely. Many patients qualify for manufacturer assistance programs that reduce out-of-pocket costs to $0 or $50 per month.
Can I use biologics if I have other health conditions?
Not always. Biologics are not recommended if you have active infections, untreated tuberculosis, heart failure, or multiple sclerosis. If you have diabetes, liver disease, or a history of cancer, your doctor will weigh risks carefully. You’ll need blood tests and screenings before starting. Always tell your dermatologist about all your health conditions and medications.
What lifestyle changes help with HS alongside biologics?
Smoking cessation is the most important. Smokers with HS respond poorly to treatment. Weight loss also helps-even a 10% reduction in body weight can reduce flare-ups. Avoid tight clothing, use gentle, fragrance-free skin products, and consider anti-inflammatory diets rich in omega-3s and vegetables. These don’t replace biologics, but they make them work better.





Jason Silva
December 21, 2025 AT 07:22