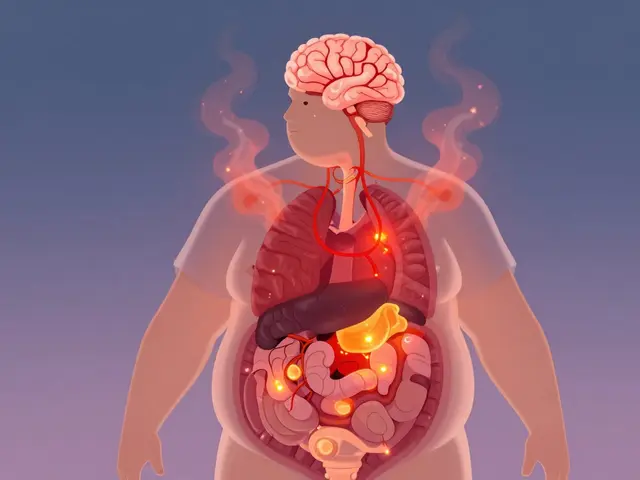
Obesity as a Chronic Disease: Understanding Metabolic Health and Realistic Weight Strategies
December 28 2025Quality Control in Pharmaceuticals: How Safe Medicines Reach Your Home
When you take a pill, you expect it to work—and to not hurt you. That’s not luck. It’s quality control, the systematic process of ensuring pharmaceutical products meet strict safety, strength, and purity standards before they leave the factory. Also known as pharmaceutical quality assurance, it’s the invisible guardrail that stops contaminated, weak, or fake drugs from ending up in your medicine cabinet. Without it, a batch of antibiotics could be too weak to fight infection, or a blood thinner might contain the wrong amount of active ingredient—both could kill you.
Quality control isn’t just about checking one sample. It’s built into every step: from how raw materials are tested, to how machines are cleaned between batches, to how each bottle gets a unique serial number. The CGMP compliance, Current Good Manufacturing Practices enforced by the FDA to ensure consistent product quality. Also known as cGMP, it’s the rulebook every drugmaker must follow is non-negotiable. If a factory fails an FDA inspection, a surprise audit where inspectors check records, equipment, and processes to confirm compliance with safety standards. Also known as FDA facility audit, it’s the main tool used to enforce drug safety, the whole batch gets held back—or worse, recalled. That’s why companies spend millions training staff, upgrading labs, and installing digital tracking systems like DSCSA track-and-trace, a U.S. system that follows every drug package from manufacturer to pharmacy using unique barcodes. Also known as drug serialization, it’s designed to stop counterfeit medicines from entering the supply chain. These aren’t buzzwords—they’re lifelines.
But quality control doesn’t end at the factory door. Even the best-made drug can become dangerous if it’s stored wrong, mixed up with another, or given to the wrong patient. That’s why hospitals use double check protocols, a safety step where two staff members independently verify high-risk medications like insulin or blood thinners before administration. Also known as independent double verification, it’s a critical barrier against human error. And when generics replace brand names, quality control ensures they’re truly equivalent—not just chemically, but in how your body responds. Studies show even tiny differences in absorption can cause serious problems with drugs like warfarin or thyroid medicine.
What you’ll find below are real stories behind those rules. You’ll read how inspections catch violations, how traceability stops fake pills, why some generic switches backfire, and how industry pressure sometimes weakens the system. These aren’t theoretical concerns—they’re the daily work of people who keep your medicine safe. Whether you’re a patient, a caregiver, or just someone who takes pills regularly, understanding quality control means you can ask better questions, spot red flags, and trust your meds more deeply.
 6 Dec
6 Dec
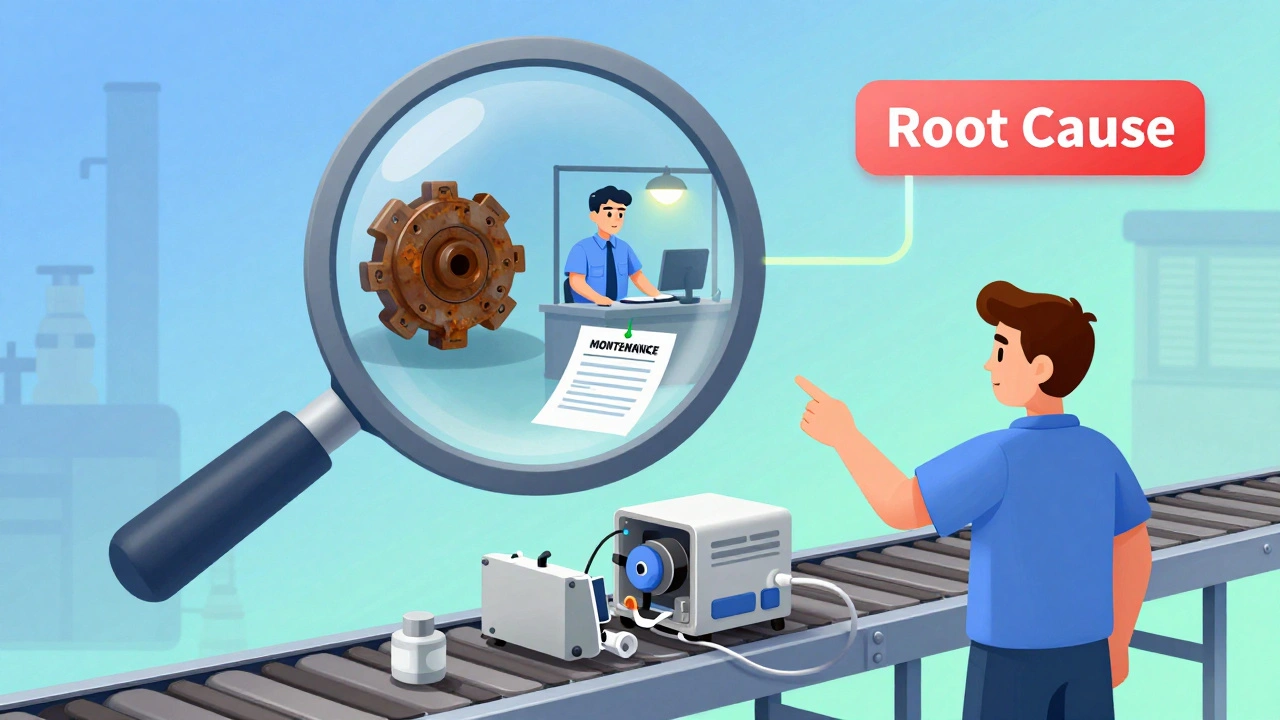
How Manufacturers Fix Quality Problems: A Practical Guide to Corrective Actions
Manufacturers fix quality problems through structured corrective actions that target root causes, not just symptoms. Learn how CAPA systems work, why most fail, and how to implement them effectively in regulated industries.
Read More...