CYP3A4 Drug Interaction Checker
Check Medication Interactions
Enter medications you're taking with Lopinavir/Ritonavir (LPV/r). This tool references the Liverpool HIV Interactions Database (2023).
Interaction Results
DANGEROUS INTERACTION
Ritonavir completely blocks CYP3A4, causing 500% higher levels of midazolam. This can lead to respiratory arrest.
Recommendation: Contraindicated - Avoid use with LPV/r. Consider alternative sedatives.
CAUTIONARY INTERACTION
Ritonavir accelerates warfarin metabolism by 300%, causing INR to drop 30%. This increases risk of blood clots.
Recommendation: Close monitoring required - Check INR weekly, adjust dose by 20-40%.
SAFE INTERACTION
Pravastatin is metabolized via non-CYP3A4 pathways. It has no significant interaction with LPV/r.
Recommendation: Can be used safely - Continue at standard dose.
This tool is for educational purposes only. Always consult your HIV specialist before making medication changes. Real-world interactions may vary based on individual metabolism, genetics, and other factors.
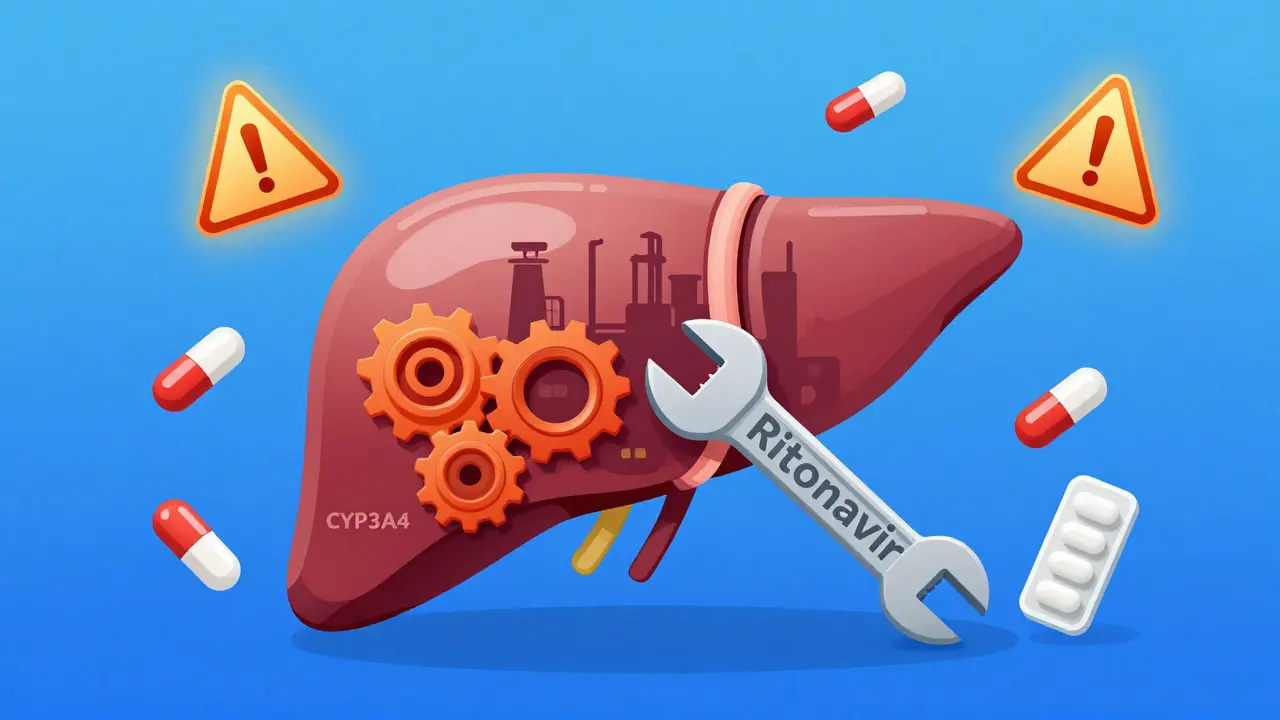
When you hear Lopinavir/Ritonavir, you might think of HIV treatment. But what most people don’t realize is that this combo isn’t just about killing the virus-it’s a high-stakes chemical balancing act that can make or break dozens of other medications you’re taking. Ritonavir, at just 100mg, isn’t there to treat HIV. It’s there to shut down your liver’s main drug-processing system: CYP3A4. And when that system goes offline, everything else you’re taking changes-sometimes dangerously.
Think of CYP3A4 like a factory worker in your liver. It’s responsible for breaking down more than half of all prescription drugs: statins, blood thinners, painkillers, even birth control. Lopinavir, the main HIV drug in this combo, gets chewed up by CYP3A4 so fast that without ritonavir, it’d be gone from your body in under 7 hours. Ritonavir doesn’t just slow it down-it smashes the factory. It binds to CYP3A4 so tightly, it permanently disables it. This is called mechanism-based inactivation. It’s not a temporary pause. It’s like throwing a wrench into the gears and welding them shut. That’s why lopinavir’s half-life jumps from 7 hours to over 12 hours. That’s also why so many other drugs suddenly become toxic.
Why Ritonavir Is the Most Potent CYP3A4 Inhibitor Out There
Other drugs try to block CYP3A4, but none do it like ritonavir. Cobicistat, the newer booster used in drugs like Prezcobix, only targets CYP3A4. Ritonavir? It’s a Swiss Army knife of enzyme disruption. It doesn’t just inhibit CYP3A4-it also shuts down CYP2D6. But here’s the twist: it turns on CYP1A2, CYP2B6, CYP2C9, and CYP2C19. That’s not a mistake. That’s by design. The result? A wild, unpredictable ride for any drug that uses those enzymes.
Take warfarin, a blood thinner. Ritonavir makes CYP2C9 work faster, so warfarin gets cleared out quicker. Your INR drops. You’re at risk of a clot. But at the same time, ritonavir slams CYP3A4, so drugs like midazolam (a sedative) build up to 500% higher levels. One patient on both drugs could go from sleepy to unresponsive in minutes. That’s why anesthesiologists have to cut midazolam doses by 80% in patients on LPV/r. Fentanyl? Same deal. Dose reductions of 60-70% are standard. Miss that, and you risk respiratory arrest.
The Interaction Explosion: 1,247 Drugs on the Radar
The Liverpool HIV Interactions Database, updated in July 2023, lists 1,247 drugs with known interactions with Lopinavir/Ritonavir. That’s more than double the number for newer regimens like darunavir/cobicistat. Why? Because ritonavir doesn’t just affect one pathway-it affects nearly every major liver enzyme.
- Tacrolimus (transplant drug): Levels spike 3-5x. Dose must be cut by 75%. Without adjustment, kidney failure is likely.
- Rivaroxaban (blood thinner): Completely contraindicated. Too much risk of internal bleeding.
- Methadone: Ritonavir speeds up its breakdown. Dose must be increased by 20-33% to avoid withdrawal.
- Statins (like simvastatin): Risk of rhabdomyolysis (muscle breakdown) increases 10-fold. Only pravastatin or rosuvastatin are considered safe.
- Hormonal contraceptives: Effectiveness drops by over 50%. Backup methods aren’t optional-they’re mandatory.
- Voriconazole (antifungal): Ritonavir induces its metabolism, making it useless. But it also inhibits other pathways, so levels can swing unpredictably. It’s contraindicated.
There’s no pattern. No simple rule. You can’t just avoid CYP3A4 substrates. You have to map out every enzyme involved. A patient on LPV/r taking metformin (safe), lisinopril (safe), and fluconazole (risky) might seem fine-but add a single dose of rifampicin (for TB), and lopinavir levels crash by 76%. Liver toxicity jumps from 11% to 33%. That’s not theoretical. That’s from a 2008 study with real patients.

Real-World Consequences: When the System Fails
Most clinicians know to check for interactions. But how many actually spend 15-20 minutes doing it? The Liverpool database gets 2.8 million hits a year. That’s not because people are curious-it’s because mistakes happen daily.
A 2022 meta-analysis found that patients on LPV/r had a 37% higher chance of stopping their HIV meds due to side effects. Why? Not because of the virus. Because of the drugs they were taking alongside it. Liver damage. Kidney stress. Heart rhythm issues. Muscle breakdown. These aren’t rare. They’re predictable.
And it’s not just HIV patients. The same interaction risks show up in people with hepatitis C, cancer, or even COVID-19. When Paxlovid (nirmatrelvir/ritonavir) hit the market, it used the same boosting trick. But the rebound effect-where viral load spikes after treatment ends-was linked to ritonavir’s long half-life. It sticks around, keeping CYP3A4 blocked, even after the antiviral is gone. That’s why some patients relapse: their body can’t clear the drug fast enough.
Why This Combo Still Exists-And Where It’s Headed
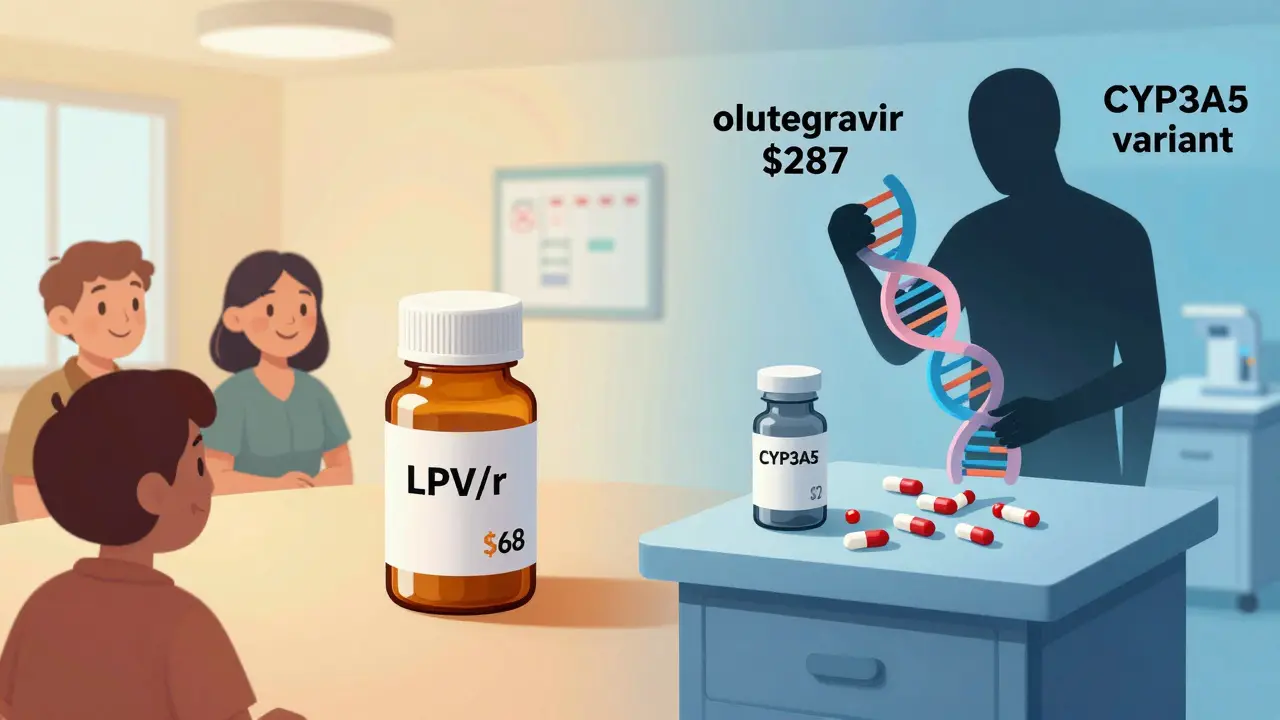
In the U.S., LPV/r is all but gone. Less than 5% of HIV patients use it. Newer drugs like dolutegravir are simpler, safer, and have fewer interactions. But in low-income countries? It’s still the backbone. Why? Cost. A full year of LPV/r costs $68 under PEPFAR programs. Dolutegravir? $287. That’s not a small difference. It’s the difference between treatment and no treatment.
But even there, the tide is turning. UNAIDS projects LPV/r will drop to 12% of first-line regimens by 2027. Why? Because the side effects are too costly. Hospitalizations. Emergency visits. Lost workdays. The long-term burden of managing these interactions is heavier than the drug’s price tag suggests.
And now, research is digging deeper. NIH-funded studies are looking at CYP3A5 gene variants. Turns out, some people naturally have more of this enzyme. They break down lopinavir faster. So even with ritonavir boosting, their levels stay low. They’re at risk of treatment failure-not because they’re noncompliant, but because of their DNA.
What You Need to Do If You’re on This Combo
If you’re prescribed Lopinavir/Ritonavir, here’s what you need to do:
- Bring every medication you take-prescription, OTC, supplements-to every appointment. Even ibuprofen. Even garlic pills.
- Ask for a full interaction screen using the Liverpool HIV Interactions Database. Don’t rely on your pharmacy app.
- If you’re on statins, ask if it’s pravastatin or rosuvastatin. If it’s simvastatin or atorvastatin, demand a change.
- If you’re on birth control, use a backup method. Always.
- Never start a new medication without checking with your HIV specialist. Not your GP. Not your pharmacist. Your HIV specialist.
- Know the signs of toxicity: unexplained muscle pain, dark urine, yellowing skin, unusual bleeding, confusion. Call your doctor immediately.
This isn’t about being careful. This is about survival. Ritonavir doesn’t just help lopinavir work. It changes how your entire body handles medicine. There’s no middle ground. Either you manage it properly-or you risk serious harm.