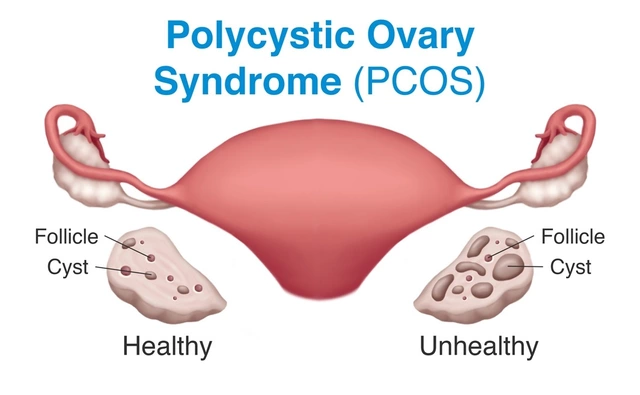
The Connection between Hirsutism and Polycystic Ovary Syndrome (PCOS)
May 17 2023FDA Approval: What It Means for Your Medications and Why It Matters
When you see FDA approval, the U.S. Food and Drug Administration’s official green light that a drug is safe and effective for its intended use. Also known as drug clearance, it’s the single most important checkpoint between a medicine being developed and ending up in your medicine cabinet. This isn’t paperwork—it’s science, testing, and oversight wrapped into one system that keeps dangerous or ineffective drugs off the market.
FDA approval doesn’t just mean a drug works. It means the company proved it works better than a placebo, that the side effects are understood and manageable, and that every pill made in every factory meets strict quality rules. That’s where FDA inspections, on-site checks of manufacturing plants to ensure they follow Current Good Manufacturing Practices come in. These aren’t surprise visits—they’re planned, risk-based audits that look at everything from clean rooms to employee training. A single failed inspection can delay approval by months, or even block a drug entirely. And it’s not just about big pharma. Generic drug makers face the same scrutiny. If a factory doesn’t pass, their version of your medication won’t get approved either.
But approval doesn’t stop at the factory. The drug supply chain, the network of manufacturers, distributors, and pharmacies that move medicine from lab to patient is now tracked end-to-end thanks to the DSCSA system. Each package gets a unique serial number so counterfeits can be caught before they reach you. And even after approval, the FDA keeps watching. If new safety issues pop up—like unexpected heart risks or dangerous interactions with common supplements—they can require label changes, issue warnings, or pull the drug entirely.
That’s why you can’t trust every online pharmacy selling "FDA-approved" pills. Some fake the label. Others sell drugs made in unapproved labs overseas. Real FDA approval means the product was made in a facility the agency has inspected and approved. It means the data behind its safety and effectiveness was reviewed by real scientists—not just a marketing team. And it means the company can’t just change the formula later without going back for approval.
What you’ll find below isn’t just a list of articles. It’s a behind-the-scenes look at how FDA approval shapes everything—from why your generic pill looks different than the brand name, to how a single typo on a prescription can cause a deadly error, to why some medications are kept behind the counter even after approval. You’ll see how industry influence sometimes bends the rules, how quality failures erode trust, and how patients are often the last line of defense. This isn’t theory. It’s real, everyday stuff that affects whether your medicine works, harms you, or does nothing at all.
 1 Dec
1 Dec
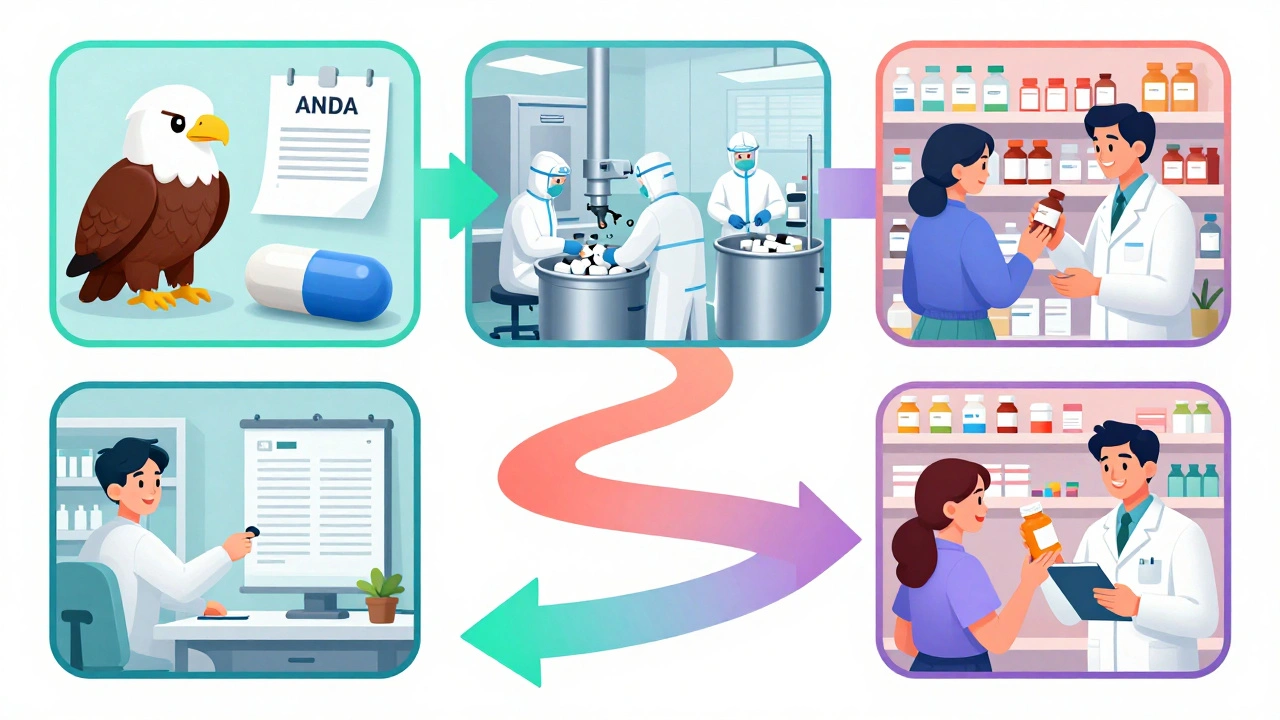
From ANDA to Shelf: How Generic Drugs Reach Retail Pharmacies
Discover how generic drugs move from FDA approval through manufacturing, payer negotiations, and distribution to reach retail pharmacies-and why this process saves Americans billions each year.
Read More...