When a brand-name drug loses its patent, a cheaper version hits the market - and suddenly, millions of people pay less for the same medicine. That’s not magic. It’s the FDA generic approvals system at work. Every year, the FDA approves hundreds of generic drugs, and each one saves the U.S. healthcare system billions. But how much? And which years delivered the biggest drops in price? The numbers tell a clear story - and they’re not what most people expect.
How the FDA Measures Generic Savings
The FDA doesn’t just count how many generics get approved. It tracks exactly how much money those new approvals save in the first year after they hit the market. This is called the first 12 months post-approval savings metric. It’s not about how much all generics saved in a year - it’s about the immediate financial shockwave when a new generic enters a market that used to be dominated by a single, expensive brand.
Here’s how it works: When a brand drug loses exclusivity, the generic version usually sells for 70% to 90% less. The FDA compares the brand’s price before the generic arrived to the price after, then multiplies that difference by how many pills were sold. They also account for the fact that even the brand-name drug often drops its price to compete. The result? A real-dollar number showing how much consumers, insurers, and government programs saved because that one new generic got approved.
This method is different from what the Association for Accessible Medicines (AAM) reports. AAM looks at the entire year’s spending on all generics - every pill, every prescription, every patient. That’s the total savings from all generics in use. The FDA’s number is more like a snapshot of new competition. Both matter. But if you want to see where the biggest savings came from in a given year, the FDA’s first-generic data is the key.
Year-by-Year Savings from New Generic Approvals
Not every year is the same. The savings from new generic approvals swing wildly depending on which drugs lose patent protection. A single blockbuster drug coming off patent can generate more savings than dozens of smaller ones.
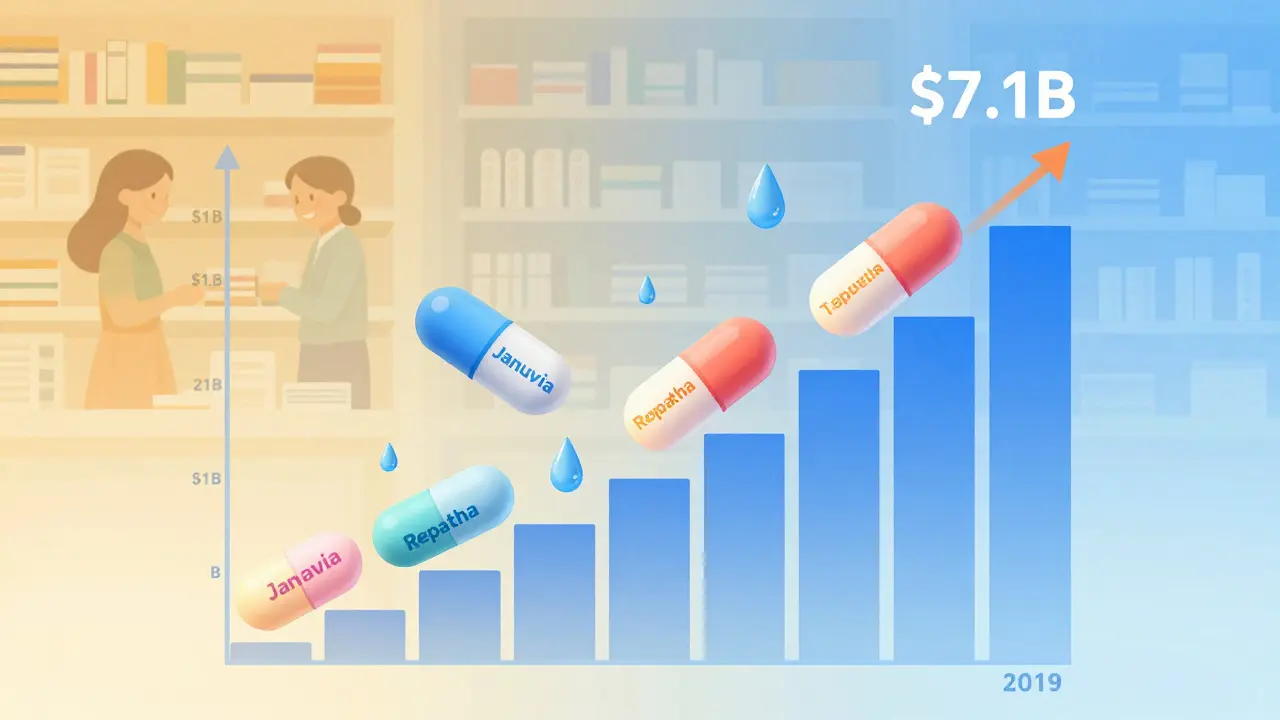
In 2018, the FDA reported $2.7 billion in savings from first-time generic approvals. That was solid, but nothing compared to what came next.
2019 was the peak year: $7.1 billion. Why? Because three major drugs - including the cholesterol med Repatha and the diabetes drug Januvia - lost patent protection all at once. Their generic versions dropped prices from over $1,000 a month to under $30. That’s not a discount. That’s a revolution.
Then came 2020: $1.1 billion. A sharp drop. No blockbuster patents expired that year. The pipeline was quiet. The savings didn’t vanish - they just paused.
2021 bounced back to $1.37 billion. Five drugs accounted for nearly half of that total. One of them, a generic version of the cancer drug Velcade, saved patients $1.2 billion in its first year alone.
2022 saw another surge: $5.2 billion. The FDA pointed to several approvals in "relatively large markets." That meant drugs used by hundreds of thousands of people - like blood pressure meds, antidepressants, and asthma inhalers - suddenly had cheaper alternatives. The total savings from all generic approvals that year (including repeat generics) hit $18.9 billion.
That’s the pattern: big spikes when major drugs go generic, quiet years when they don’t. There’s no steady climb. It’s a rollercoaster shaped by patent cliffs, not policy.
Total Generic Savings: The Bigger Picture
If you only look at new approvals, you miss the full impact. The real power of generics is in their cumulative effect. In 2023, generics and biosimilars saved the U.S. healthcare system $445 billion - that’s nearly half a trillion dollars in one year.
That number comes from the Association for Accessible Medicines. It’s not just about new drugs. It’s about every generic pill filled that year. In 2023, 90% of all prescriptions in the U.S. were for generics. Yet those generics made up only 13.1% of total drug spending. The rest? Brand-name drugs - which cost 7 to 10 times more.
Medicare saved $137 billion. Commercial insurers saved $206 billion. Medicaid saved the rest. In California alone, generics saved over $37 billion. In Alaska, it was $354 million. The savings scale with population, but the impact is universal.
Therapeutic areas show where the biggest wins happened: heart disease saved $118 billion, mental health saved $76 billion, cancer saved $25 billion. These aren’t abstract numbers. They’re people who can afford their meds now. People who don’t skip doses because of cost. People who aren’t choosing between insulin and groceries.

Why Some Savings Don’t Reach Patients
Here’s the catch: just because a drug is cheaper doesn’t mean patients pay less. Pharmacy benefit managers (PBMs) control most of the pricing between insurers, pharmacies, and manufacturers. A 2023 Senate Finance Committee report found that only 50% to 70% of generic savings actually make it to the patient’s copay.
Why? PBMs take rebates from drugmakers. Sometimes, those rebates are higher for brand-name drugs than generics. So pharmacies get paid more to push the expensive version - even when a cheaper generic exists. It’s a broken incentive system.
Still, patients feel the difference. The average generic copay is $6.97. For many, it’s $5 or even $0 at Walmart, Costco, or CVS. Pharmacists say 92% of generic prescriptions are filled for under $20. That’s life-changing for someone on a fixed income.
State Medicaid programs have seen massive savings. California’s Medi-Cal program saved $23.4 billion in a single year from generics. That’s money that went back into care for low-income families, not into corporate profits.
What’s Driving the Growth
The pipeline is still full. Over 600 generic applications get approved each year. The FDA’s Generic Drug User Fee Amendments (GDUFA) have cut review times - 95% of applications are reviewed in under 10 months now. That means faster access to cheaper drugs.
More blockbuster drugs are coming off patent. Drugs like Humira, Enbrel, and Keytruda are next in line. Their generics could save tens of billions over the next five years.
Biosimilars - the generic version of biologic drugs - are starting to make an impact. As of August 2024, the FDA had approved 59 biosimilars. They’re more complex to make than traditional generics, so they’re slower to enter the market. But they’re cheaper. And they’re growing.
By 2033, U.S. generic drug revenue is projected to hit $131.8 billion. That’s not because people are buying more drugs. It’s because more drugs are cheaper.
What’s Holding Back Even More Savings
There are still roadblocks. Brand-name companies use legal tricks to delay generics - like filing endless patent lawsuits, or using risk evaluation and mitigation strategies (REMS) to block generic manufacturers from getting samples for testing. The FDA’s 2023 Drug Competition Action Plan is trying to shut these practices down.
Some drugs are too complex to copy easily. Injectables, inhalers, and topical creams are harder to replicate than a simple pill. That’s why the FDA is working on new guidelines for complex generics. If they succeed, more of these drugs will go generic - and more people will save.
But the biggest challenge isn’t science. It’s money. The pharmaceutical industry spends billions lobbying to protect high prices. The system is designed to favor brand-name profits - not patient savings.
What This Means for You
If you’re taking a prescription, ask your pharmacist: "Is there a generic?" If you’re paying more than $50 a month for a common medication, there probably is. And if your insurance won’t cover it, check prices at Walmart, Costco, or GoodRx. You might pay less than your copay.
The savings from FDA generic approvals aren’t just numbers on a spreadsheet. They’re people who can afford their insulin. Parents who don’t have to choose between medicine and rent. Seniors who can keep taking their heart pills without going broke.
Every time a new generic gets approved, someone’s life gets a little easier. And the data shows - it’s happening every year. Even when the headlines don’t mention it.
How much do generic drugs save the U.S. healthcare system each year?
In 2023, generics and biosimilars saved the U.S. healthcare system $445 billion. That’s according to the Association for Accessible Medicines. This includes savings from all generics in use that year, not just newly approved ones. The FDA’s annual savings from new generic approvals - the immediate impact of each new drug entering the market - ranged from $1.1 billion to $7.1 billion per year between 2018 and 2022, depending on which major drugs lost patent protection.
Why do generic drug savings vary so much from year to year?
Savings jump or drop based on which brand-name drugs lose patent protection. If a blockbuster drug like Januvia or Repatha goes generic, it can generate billions in savings in one year. But if no major drugs expire, the savings drop sharply. It’s not a steady trend - it’s a lottery. The timing of patent expirations drives the spikes.
Do patients actually pay less when generics are approved?
Sometimes, but not always. While generics cost far less to produce, pharmacy benefit managers (PBMs) often take rebates that don’t fully pass savings to patients. A 2023 Senate report found only 50-70% of generic savings reach consumers. Still, the average generic copay is $6.97, and many are under $20 - a huge drop from brand-name prices that can exceed $1,000 a month.
What’s the difference between FDA and AAM savings numbers?
The FDA measures savings from drugs approved in a given year - only the first 12 months after approval. The AAM measures total savings from all generics used in a calendar year. So the FDA number shows the impact of new competition; the AAM number shows the full weight of generics in the market. In 2022, FDA reported $5.2 billion from new approvals, but AAM reported $408 billion in total savings from all generics.
Are biosimilars saving as much as traditional generics?
Not yet. As of August 2024, the FDA had approved 59 biosimilars - the generic versions of complex biologic drugs like Humira and Enbrel. But biosimilars are harder and more expensive to make, so they’re slower to enter the market and usually cost more than traditional generics. Their savings are growing, but they still make up a small fraction of total generic savings. That’s expected to change as more biosimilars get approved over the next decade.
How can I find out if my prescription has a cheaper generic?
Ask your pharmacist. They know which drugs have generics and can check prices at different pharmacies. You can also use tools like GoodRx, SingleCare, or Blink Health to compare cash prices. Many common generics - like metformin, lisinopril, or atorvastatin - cost under $10 for a 30-day supply at Walmart or Costco, even without insurance.






Kuldipsinh Rathod
December 26, 2025 AT 06:33