When you pick up a prescription at the pharmacy, you might be handed a generic version instead of the brand-name drug you expected. That’s not a mistake-it’s by design. The U.S. Food and Drug Administration (FDA) ensures these generics are safe and effective through a system most people never see: the FDA Orange Book. But how do you know if the generic you’re being given is truly interchangeable with the brand? It’s not enough to assume they’re the same. You need to check the Orange Book-and here’s how to do it right.
What the FDA Orange Book Actually Is
The FDA Orange Book isn’t a physical book anymore. It’s an online database called the Approved Drug Products with Therapeutic Equivalence Evaluations. First published in 1980 and updated daily since 2007, it’s the official source that tells pharmacists, doctors, and patients which generic drugs can be swapped for brand-name versions without losing effectiveness or safety. It’s not just a list of drugs. It’s a legal and scientific tool created under the Hatch-Waxman Act of 1984. That law was designed to speed up access to cheaper generics while protecting innovation. The Orange Book does this by clearly marking which generics are approved as therapeutically equivalent to their brand-name counterparts. Without it, pharmacies couldn’t legally substitute drugs in most states. The database covers over 16,000 approved drug products, including both prescription and over-the-counter (OTC) medications. But here’s the catch: only prescription drugs get therapeutic equivalence ratings. OTC drugs are listed, but they don’t have codes like AB or BX-because they’re not evaluated for substitution.The Therapeutic Equivalence Code: AB, BX, and What They Mean
The heart of the Orange Book is its two-letter TE code. This code tells you whether a generic can be substituted for the brand. If you see an AB rating, that’s the green light. It means the generic has been proven to be pharmaceutically and bioequivalent to the reference drug. That’s the gold standard. Here’s what the codes actually mean:- AB: Therapeutically equivalent. Safe to substitute. This is what you want.
- AB1, AB2, AB3: Also equivalent, but to different reference drugs. For example, if two brand drugs exist for the same active ingredient, generics are rated against each one separately.
- BX: Not equivalent. These drugs have issues-maybe inconsistent absorption, different release patterns, or unproven bioequivalence. Don’t substitute these.
- No code: The product is either discontinued or an OTC drug. It’s not rated.
- It’s approved as safe and effective.
- It has the same active ingredient, strength, dosage form, and route of administration as the brand.
- It’s bioequivalent-meaning it gets into your bloodstream at the same rate and amount.
- Its labeling matches the brand’s.
- It’s made under FDA-approved manufacturing standards.
How to Search the Electronic Orange Book Step by Step
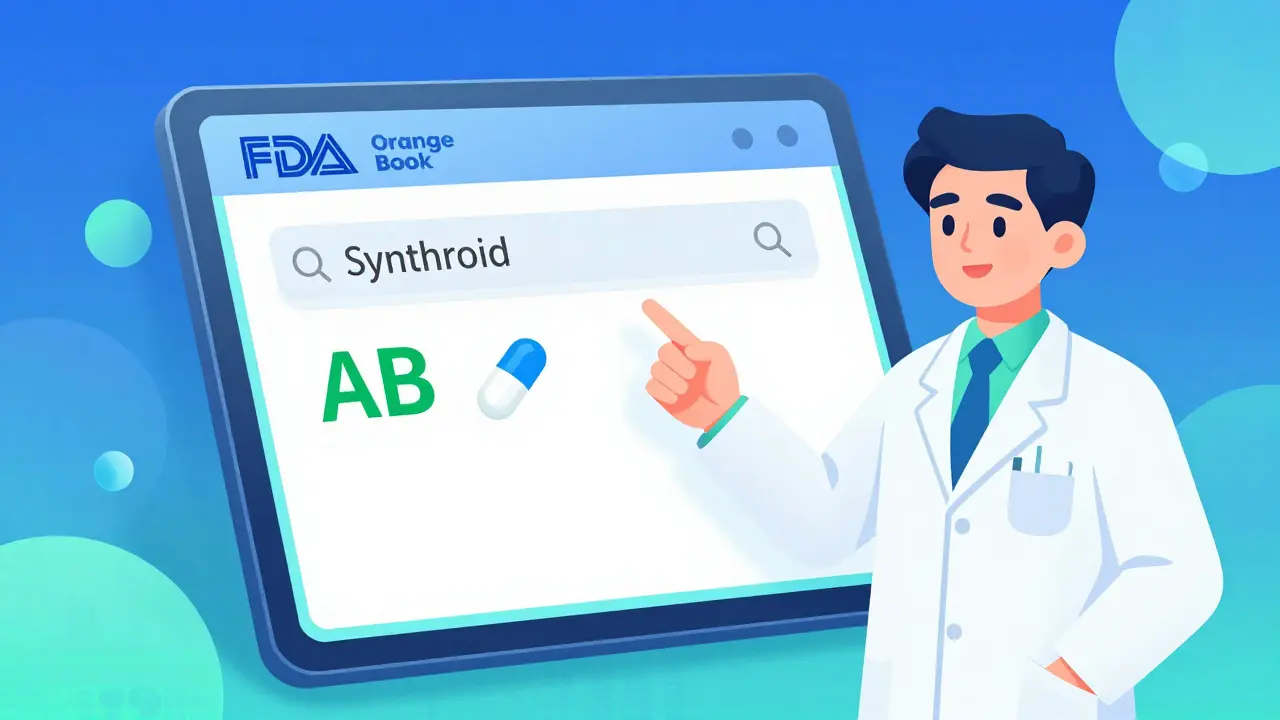
The FDA’s Electronic Orange Book is free, public, and updated daily. Here’s how to use it correctly.- Start with the brand name. Go to the search page and type in the brand name-like "Synthroid" or "Lipitor". Don’t use the generic name yet. You’re looking for the reference listed drug (RLD).
- Find the active ingredient. Once you get results, look for the "Active Ingredient" column. For Synthroid, that’s levothyroxine sodium.
- Use the Ingredient Search. Click on the active ingredient to see every product that contains it. This will show you all approved versions, brand and generic.
- Filter by dosage form and route. If you’re checking a pill, select "Tablet, Oral". If it’s an inhaler, pick "Inhalation, Powder". Don’t mix forms-equivalence only applies within the same delivery method.
- Identify the RLD. In the results, look for the column labeled "RLD". The brand-name drug will say "Yes". All the generics will say "No".
- Check the TE Code. The last column shows the rating. If it says "AB", that generic is approved for substitution. If it says "BX", it’s not. If there’s no code, it’s not rated.
Why This Matters in Real Life
You might think, "If it’s the same chemical, why does it matter?" But biology isn’t that simple. Take levothyroxine, used for thyroid conditions. Even tiny differences in absorption can cause serious problems-fatigue, weight gain, heart palpitations. The FDA has found that some generics, even if AB-rated, may behave differently in sensitive patients. That’s why many states require a doctor’s approval before substituting levothyroxine, even if the Orange Book says it’s fine. A 2022 survey of 1,250 pharmacists showed that 68% check the Orange Book weekly. But 42% still struggle with complex products like inhalers, topical creams, or injectables. Why? Because bioequivalence is harder to prove for these forms. A cream might look identical, but if the active ingredient doesn’t penetrate the skin the same way, it won’t work the same. Also, patent listings in the Orange Book can delay generics. Brand companies sometimes file patents on minor changes-like a new coating or packaging-to extend exclusivity. The FDA doesn’t judge whether these patents are valid, only that they’re listed. That’s why some generics take years to appear, even after the original patent expires.What the Orange Book Doesn’t Tell You
The Orange Book is powerful-but it’s not the whole story. First, it doesn’t show state laws. In some states, pharmacists can’t substitute certain drugs without a doctor’s OK-even if the Orange Book says AB. This is common for narrow therapeutic index drugs like warfarin, digoxin, or lithium. Second, it doesn’t track real-world performance. A drug might be AB-rated based on clinical trials, but if patients report side effects or inconsistent results, that’s not in the database. Third, third-party sites like Drugs.com or IBM Micromedex pull data from the Orange Book-but they’re often 24 to 72 hours behind. If you’re verifying a drug for a patient today, always go to the FDA’s site directly. And finally, discontinued products still show up in the database. They’re marked with a "Discontinued Drug Product List" tag. Don’t confuse those with active drugs.
What to Do If You’re Still Unsure
If you’re a patient and you’re worried about a substitution, ask your pharmacist. They’re trained to use the Orange Book. If you’re a provider, double-check the TE code before prescribing or authorizing a switch. The FDA offers free tools to help:- A 12-page Quick Reference Guide (updated March 2023)
- Bi-monthly Drug Info Rounds webinars with live Q&A
- Email support at [email protected] usually come within 48 business hours
Final Thought: The Orange Book Is Your Shield
Generic drugs save the U.S. healthcare system over $300 billion a year. But that savings only works if the substitutes are truly equivalent. The FDA Orange Book is the only official tool that gives you that assurance. Don’t rely on assumptions. Don’t trust third-party sites. If you need to know whether a generic can be swapped for a brand, go straight to the source. Search the brand name. Find the active ingredient. Check the TE code. If it’s AB, you’re good. If it’s not, ask why. Because when it comes to your health-or your patient’s health-there’s no room for guesswork.Is the FDA Orange Book free to use?
Yes, the Electronic Orange Book is a free, public database maintained by the U.S. Food and Drug Administration. You can access it anytime at https://www.accessdata.fda.gov/scripts/cder/ob/. No registration, login, or payment is required.
Can I substitute any generic labeled "AB" without checking anything else?
Not always. While an AB rating means the FDA considers the generic therapeutically equivalent, state laws may restrict substitutions for certain drugs-especially those with a narrow therapeutic index like levothyroxine, warfarin, or phenytoin. Always check your state’s pharmacy regulations before substituting.
Why do some generics have AB1, AB2, or AB3 ratings?
When a drug has more than one reference listed drug (RLD)-meaning multiple brand-name versions were approved over time-each gets its own equivalence group. AB1 means the generic matches RLD1, AB2 matches RLD2, and so on. Substituting a generic rated AB1 for a brand that’s RLD2 could lead to ineffective treatment.
Are over-the-counter (OTC) drugs listed in the Orange Book?
Yes, OTC drugs appear in the database, but they do not receive therapeutic equivalence (TE) ratings. The FDA only evaluates prescription drugs for substitution. So if you see an OTC drug in the Orange Book with no AB or BX code, it’s listed for informational purposes only.
How often is the Orange Book updated?
The Electronic Orange Book is updated daily with new approvals, patent changes, and discontinuations. However, major revisions-like full reclassifications or format changes-happen monthly. Always verify you’re viewing the most current version when doing critical checks.
Can I trust third-party apps like Drugs.com or Micromedex for Orange Book data?
They’re convenient, but not reliable for critical decisions. These tools pull data from the FDA, but they can be 24 to 72 hours behind. For legal, clinical, or insurance purposes, always use the official FDA website to confirm therapeutic equivalence ratings.
What does a BX rating mean for a generic drug?
A BX rating means the FDA has determined the generic is not therapeutically equivalent to the reference drug. This could be due to bioequivalence issues, inconsistent absorption, or unproven performance. These drugs should not be substituted for the brand unless directed by a prescriber.
Do all generic drugs need to be rated in the Orange Book?
No. Only prescription drugs approved through the Abbreviated New Drug Application (ANDA) process receive TE ratings. Some generics may be approved but not rated if they’re for unapproved uses, have incomplete data, or are discontinued. Always check the TE code column-no code means no FDA equivalence determination.



Kimberly Mitchell
January 13, 2026 AT 01:16